Our Pipeline
Our Pipeline
Currently in various stages of development and growth, our product portfolio is built upon botanical compounds that have the potential to address worldwide unmet medical demands.
Responsible Jaguar Entity

Noninfectious diarrhea in adults with HIV/AIDS on antiretroviral therapy*
Cancer therapy–related diarrhea (CTD)*
Phase 3 trial ongoing globally
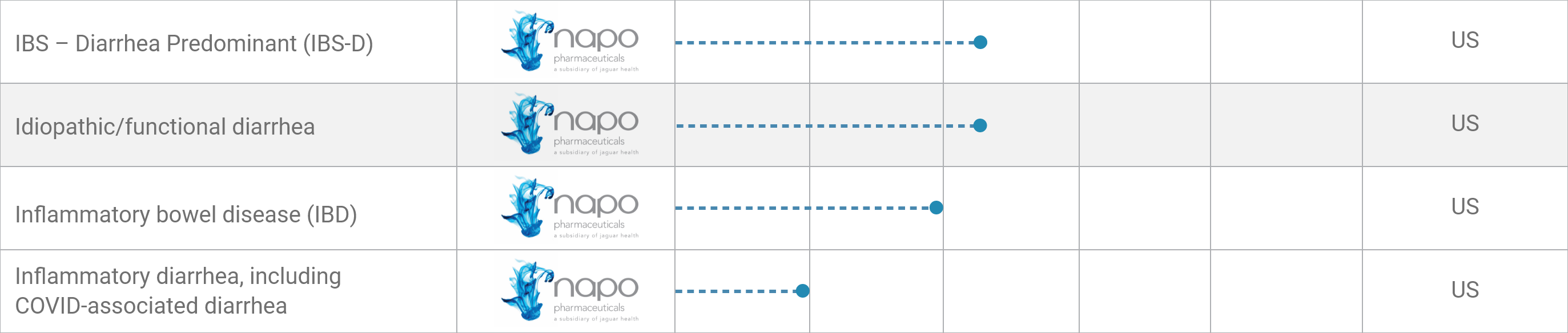
IBS – Diarrhea Predominant (IBS-D)*
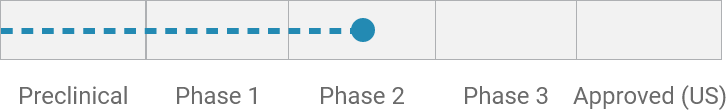
Idiopathic/functional diarrhea*
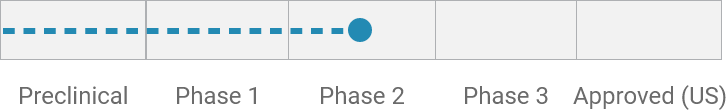
Inflammatory bowel disease (IBD)*

Inflammatory diarrhea,
including COVID-associated diarrhea
Geographic focus of clinical activity: EU and US

Responsible Jaguar Entity

Rare disease: Short Bowel Syndrome with Intestinal Failure (SBS-IF) & Congenital Diarrheal Disorders (CDD)
Geographic focus of clinical activity: EU, US, & Middle East

SBS-IF is initial focus of Napo Therapeutics for conditional approval; Crofelemer has orphan-drug designation in the US and European Union for SBS
Responsible Jaguar Entity

Symptomatic relief of diarrhea from cholera*

Received preclinical services funded by the National Institute of Allergy and Infectious Diseases for toxicity studies
Animal Health Product Portfolio
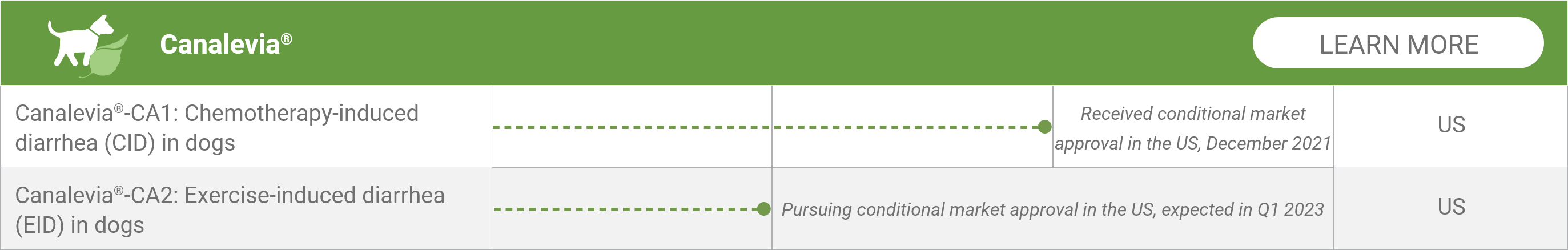
Canalevia®-CA1: Chemotherapy-induced diarrhea (CID) in dogs*

Received conditional market approval in the US, December 2021
Canalevia®-CA2: Exercise-induced diarrhea (EID) in dogs*

Pursuing conditional market approval in the US, expected in Q1 2023
*Geographic focus of clinical activity: US